In the fitness, weight training and bodybuilding community, it is well known that protein is not only an essential nutrient, but also one that supports the development of muscle mass and strength. Therefore, the intake of 1.6 to 2.2 grams per kilogram of body weight per day is considered a scientific recommendation in these circles [1]. Nevertheless, opinions differ as to whether animal or vegetable protein is better or where the differences lie.
When most of us think of protein sources, the first things that come to mind are meat, fish, dairy products, eggs and protein powders. But as statistics show, the percentage of people in the population who classify themselves as vegans, or people who largely avoid animal products, is increasing significantly. We can also see an increase in vegan people in fitness, weight training and bodybuilding circles. Many of them would certainly argue that plant-based protein is healthier and better for building muscle.
Especially in light of the fact that avowed vegans, but also die-hard meat eaters, tend to vehemently defend their point of view, a discussion about whether animal or plant protein is healthier or more effective for muscle building can quickly get out of hand. Therefore, we feel it is necessary to calm the minds on both sides right at the beginning of this article. This is not meant to be an article about ethical viewpoints, nor is it meant to defend either side. Instead, it is meant to serve as an objective discussion of the current scientific data that has examined the differences between animal and plant protein sources and how they affect muscle building.
Therefore, we will not go into greater detail here regarding potential health factors, spiritual effects, or influences on climate change. That discussion is well beyond the scope of this article. At this point, we are solely concerned with muscle growth. As always, we want to encourage healthy, data-based discussion and welcome any comments as long as they are not offensive. However, if you feel particularly inclined to either side, please take the time to rationally present your response without merely defending personal opinions, beliefs, or weak evidence. We are not trying to convince anyone of anything, but merely to present the scientific data.
What factors influence muscle protein synthesis?
The balance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB) determines whether we build up or break down muscle in the long term, or whether our muscle mass remains the same. Build-up and break-down processes are constantly taking place in our bodies. Most cells have a certain “shelf life”. This means that after a certain time they lose their function and need to be replaced. If the rate of muscle protein synthesis exceeds the rate of muscle protein breakdown in the long term, the bottom line is that we build muscle. A negative protein balance, on the other hand, leads to muscle mass breakdown in the long term. The following graphic shows a protein balance that leads to the preservation of muscle mass [3].
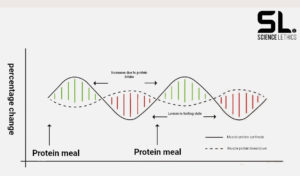
In protein balance, muscle protein breakdown (MPB) and muscle protein synthesis (MPS) counterbalance each other. In contrast, to build muscle mass, we need to increase the rate of MPS over MPB [3].
There are essentially two major factors that positively influence MPS. The first, as shown, is dietary intake, especially in the form of protein [1]. The second important pillar is strength training [4]. Since strength training in most cases also involves minute injuries to muscle cells, it also increases the rate of muscle protein breakdown [4]. Thus, depending on the training volume and intensity, as well as recovery, muscle protein breakdown can be increased as much or more than muscle protein synthesis, which is why too much training and too little recovery can actually hinder muscle building in the long run.
In terms of dietary intake, MPS is particularly influenced by the presence of essential amino acids (EAAs). In particular, the essential amino acid leucine is considered to trigger the mTOR signaling pathway, which is significantly involved in muscle protein synthesis [5]. Based on the current state of studies, it appears that an amount of about three grams of leucine within a complete dietary protein, i.e., in combination with all other 19 proteinogenic amino acids, represents the minimum dose that leads to the maximum increase in MPS [6]. Accordingly, most comparisons between animal and plant protein sources relate to their leucine content. While the levels of leucine and the other EAAs play an important role in stimulating MPS, we must first include other factors.
The quality and anabolic potential of protein sources
Protein quality is strongly dependent on the EAA and leucine content of a protein source, whether it is animal or plant protein. Until recently, these were evaluated using the so-called PDCAAS scale. The abbreviation stands for “Protein Digestibility Corrected Amino Acid Score,” which translates as amino acid score corrected for protein digestibility. However, this system has some limitations and was therefore replaced by the DIAAS, which means Digestible Indispensable Amino Acid Score [7].
Both methods are used to measure what amount of a particular protein source is necessary to avoid protein deficiency [8]. Avoiding a deficiency is certainly not the same as maximizing muscle gain. Therefore, the two systems are not 100 percent applicable to muscle building for us. While they are good for evaluating the quality and bioavailability of protein sources, they do not necessarily correlate with the anabolic potential of a protein-rich food [8]. For example, soy protein and beef protein rank similarly on the PDCAAS scale, but studies have concluded that beef increases muscle protein synthesis more than soy protein [8, 9].
The anabolic potential of a protein source depends on several factors (digestibility, composition, and absorption kinetics of amino acids), of which leucine seems to be the most important [8, 10, 11, 12]. So how do animal and plant proteins compare in terms of protein quality and anabolic potential? In terms of their essential amino acid content, animal showed a higher percentage of total protein than plant protein [13]. When interpreting the following graph, it should be noted that the analysis does not take into account the essential amino acid tryptophan, as it must be determined via a separate procedure. However, since plant protein generally also contains less tryptophan than animal protein sources, the basic message of this graph remains valid [14].
Compared to plant protein sources, animal protein sources have a higher content of essential amino acids. The analysis presented here did not include the essential amino acid tryptophan [13].
With regard to protein kinetics, studies show that plant protein sources are less well digested than animal sources [15]. As a rule, this is measured by the proportion of amino acids that are digested and absorbed and are thus available for protein synthesis in the body. Animal sources of protein have values of over 90 percent, whereas foods that provide plant protein achieve between 45 and 80 percent [15]. Isolated forms of plant protein, such as protein powders based on soy, rice, or peas, can often also be digested and absorbed at 90 percent and above because substances such as phytic acid and dietary fiber, which can interfere with digestion, have been removed in the process of preparation [8, 15].
Amino acids from plant foods are converted to urea at a higher rate than animal protein [16]. This decreases the potential of amino acids to increase MPS and contribute to muscle development. The reason for this is likely the lower proportion of leucine and other key amino acids that initiate the process of MPS. This leaves more non-essential amino acids that cannot be utilized by the MPS and oxidize in the liver. This theory has been at least partially confirmed by studies showing that soy protein leads to higher oxidation of amino acids than milk protein when both are consumed in equal amounts [17]. In other words, protein from plant sources can be used to a lesser extent for muscle protein synthesis if we consider them alone [8].
All of the factors discussed are accounted for in the DIAAS, which shows lower values overall for plant protein than for animal protein sources [7, 18].
The DIAAS evaluates the digestibility of the indispensable amino acids and is the currently valid marker for protein quality. Animal protein sources perform better than vegetable protein [19].
Animal and plant protein in real life
So far, we have discussed only the background and theories that we can use to evaluate the difference between animal and plant sources of protein. Now it’s time to put it into practice. Research has shown that acute intake of soy protein stimulates protein synthesis less than Whey protein, skim milk, or beef [20, 21, 22]. Other plant protein sources have been significantly less studied. Therefore, any statements about other forms compared to Whey and co. should be considered with caution so far.
This acute increase in MPS also carries over to long-term muscle building. Milk proteins, for example, have been shown to be more effective than soy protein in increasing lean body mass when both are taken in equal amounts and within the same time frame [23, 24]. However, other research shows that plant-based protein can also be effective for building muscle. The only catch is that you have to take more of it.
Studies in which participants were given higher amounts of milk-based protein or plant-based protein showed similar long-term muscle gain between the two approaches [25, 26]. This is because at least 30 grams of pure plant-based protein was consumed in both studies. Although plant protein sources contain lower levels of leucine and EAAs, when consumed in higher amounts, they still reach the threshold of three grams of leucine discussed at the beginning of this article to maximally stimulate MPS. No matter how high quality the protein source, intake of more than three to four grams of leucine per meal does not appear to significantly increase MPS further [6].
Since plant protein sources generally contain less leucine and EAAs than animal protein, a higher amount of them must be ingested if the goal is to reach the leucine threshold of around three grams to maximize MPS.
Conclusion and summary
Animal protein appears to be superior to plant protein in terms of muscle protein synthesis. It has more essential amino acids as well as leucine on a percentage basis and is more effectively digested and absorbed by the body. However, this problem can be circumvented in a plant-based diet by consuming a higher amount of the plant-based protein sources and thus achieving a higher absolute intake of the important amino acids. Also, combining different plant protein sources can help to complement each other’s amino acid profiles in a positive way.
However, if not enough protein is supplied as part of a plant-based diet to balance these factors, optimal muscle building cannot take place. In practice, this often proves difficult because plant-based protein sources, with the exception of protein powders, contain less protein in absolute terms and in relation to carbohydrates and fats. Certainly, a vegan diet is thus not ideal for muscle building. Nevertheless, it is understandable that some athletes would like to eat less or no animal foods for ethical reasons.
You need proper guidance for your training comeback?
Do you already know our specially designed COMEBACK FITYEAR 2021 Training Programs? 4 dedicated program guides for beginner, as well as intermediate to advanced trainees, who want to become physical active again – at home or at the gym- after a layoff period. We wrote the COMEBACK FITYEAR 2021 Programs, so you can get back in shape – after a break, especially during those pandemic lockdown times. In the comfort of your own home, your own home gym or a commercial fitness club – we got you covered: from solely bodyweight based exercises to simple workout equipment like dumbbells and resistance bands up to a proper gym setup: this is exactly what you need to get a great and effective workout again, while still being able to progress and build muscle!
We are looking forward to your visit on our website. Click here and learn more now.
READY TO TRAIN LIKE A PRO?
LET’S GET YOUR GAINS BACK. TOGETHER.
____________________________________________________
Primary source: Charlie Ottinger: “Plant vs. Animal Protein”, www.themusclephd.com
References:
- Iraki, Juma, et al. “Nutrition Recommendations for Bodybuilders in the Off-Season: A Narrative Review.” Sports 7.7 (2019): 154.
- Statista: “Personen in Deutschland, die sich selbst als Veganer einordnen oder als Leute, die weitgehend auf tierische Produkte verzichten, in den Jahren 2016 bis 2020”, de.statista.com, gesehen am 11.11.2020
- Burd, Nicholas A., et al. “Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences.” Journal of applied physiology 106.5 (2009): 1692-1701.
- Wackerhage, Henning, et al. “Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise.” Journal of Applied Physiology 126.1 (2019): 30-43.
- Lane, Michael T., et al. “Endocrine responses and acute mTOR pathway phosphorylation to resistance exercise with leucine and whey.” Biology of sport 34.2 (2017): 197.
- Morton, Robert W., Chris McGlory, and Stuart M. Phillips. “Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy.” Frontiers in physiology 6 (2015): 245.
- Leser, S. “The 2013 FAO report on dietary protein quality evaluation in human nutrition: Recommendations and implications.” Nutrition Bulletin 38.4 (2013): 421-428.
- van Vliet, Stephan, Nicholas A. Burd, and Luc JC van Loon. “The skeletal muscle anabolic response to plant-versus animal-based protein consumption.” The Journal of nutrition 145.9 (2015): 1981-1991.
- Phillips, Stuart M. “Nutrient-rich meat proteins in offsetting age-related muscle loss.” Meat science 92.3 (2012): 174-178.
- Koopman, Rene, et al. “Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein.” The American journal of clinical nutrition 90.1 (2009): 106-115.
- Pennings, Bart, et al. “Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men.” The American journal of clinical nutrition 93.5 (2011): 997-1005.
- Volpi, Elena, et al. “Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults.” The American journal of clinical nutrition 78.2 (2003): 250-258.
- Gorissen, Stefan HM, et al. “Protein content and amino acid composition of commercially available plant-based protein isolates.” Amino acids 50.12 (2018): 1685-1695.
- Joint, W. H. O. “Protein and amino acid requirements in human nutrition.” World health organization technical report series 935 (2007): 1.
- Consultation, FAO Expert. “Dietary protein quality evaluation in human nutrition.” FAO Food Nutr. Pap 92 (2011): 1-66.
- Fouillet, Hélène, et al. “Absorption kinetics are a key factor regulating postprandial protein metabolism in response to qualitative and quantitative variations in protein intake.” American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 297.6 (2009): R1691-R1705.
- Yang, Yifan, et al. “Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men.” Nutrition & metabolism 9.1 (2012): 1-9.
- Rogerson, David. “Vegan diets: practical advice for athletes and exercisers.” Journal of the International Society of Sports Nutrition 14.1 (2017): 36.
- Phillips, Stuart M. “Current concepts and unresolved questions in dietary protein requirements and supplements in adults.” Frontiers in Nutrition 4 (2017): 13.
- Phillips, Stuart M. “The science of muscle hypertrophy: making dietary protein count.” Proceedings of the nutrition society 70.1 (2011): 100-103.
- Tang, Jason E., et al. “Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men.” Journal of applied physiology (2009).
- Wilkinson, Sarah B., et al. “Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage.” The American journal of clinical nutrition 85.4 (2007): 1031-1040.
- Hartman, Joseph W., et al. “Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters.” The American journal of clinical nutrition 86.2 (2007): 373-381.
- Volek, Jeff S., et al. “Whey protein supplementation during resistance training augments lean body mass.” Journal of the American College of Nutrition 32.2 (2013): 122-135.
- Brown, Erin C., et al. “Soy versus whey protein bars: effects on exercise training impact on lean body mass and antioxidant status.” Nutrition Journal 3.1 (2004): 22.
- Joy, Jordan M., et al. “The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance.” Nutrition journal 12.1 (2013): 1-7.